(This first appeared as my column in www.multiplesclerosisnewstoday.com)
There is some good news about stem cell therapy and some that’s not so great.
A just-published study concludes that one form of human stem cell therapy is more effective at treating multiple sclerosis than the best of the MS medications being used currently.
The not-so-good news is that approval of this therapy in the U.S. still seems to be a long way off.
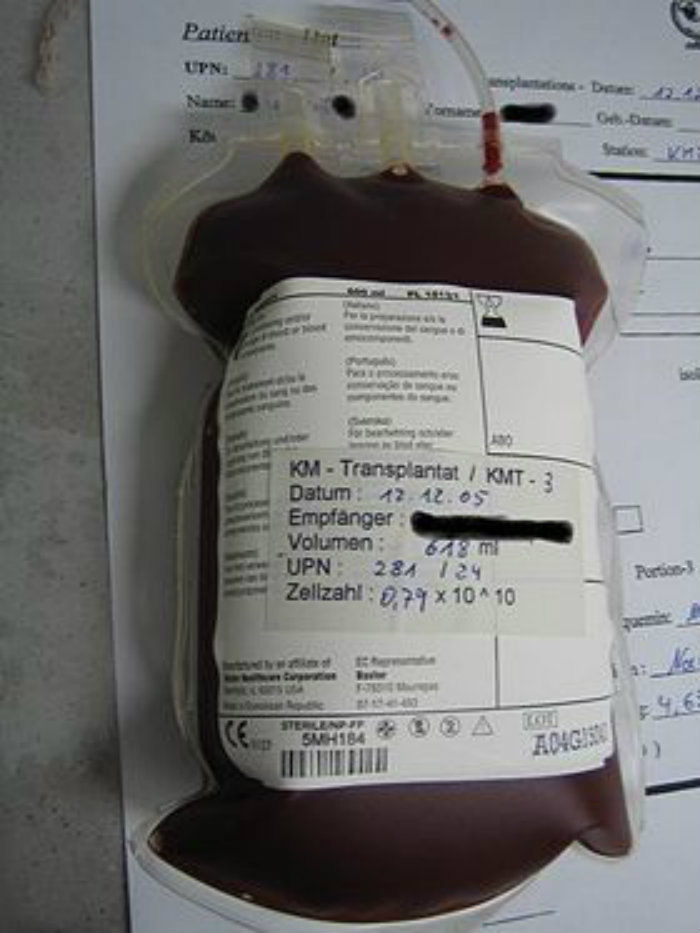
The treatment is known as high-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT). The procedure, more widely known as HSCT, aims to suppress active disease and to prevent further disability by removing disease-causing cells and resetting the immune system. During the procedure doctors collect a patient’s blood-forming stem cells, give the patient high-dose chemotherapy to deplete the immune system, and then return the patient’s own stem cells to rebuild the immune system.
Stem cell replacement is better than MS drugs
The five-year study, that was published in the February issue of Neurology, shows that HDIT/HCT can result in sustained remission of relapsing-remitting MS. Five years after receiving HDIT/HCT, 69% of the trial participants had no progression of disability, relapse of MS symptoms or new brain lesions. And some of the patients had some of their symptoms improve. This occurred without taking any MS medications after the stem cells were replaced.
“These extended findings suggest that one-time treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications for people with a certain type of MS,” said Anthony S. Fauci, MD, the Director of the National Institute of Allergy and Infectious Diseases.
More study needed
But, here’s the not-so-good news. In a press release Fauci continued: “These encouraging results support the development of a large, randomized trial to directly compare HDIT/HCT to standard of care for this often-debilitating disease.” So, Dr. Fauci, how many more years will that take? Granted, the study that was just completed involved only 24 volunteers, all of whom had aggressive, relapsing-remitting MS. We know the treatment carries some risks and that many participants in the study experienced some serious, but expected, side effects, such as infections. But, five years after receiving HDIT/HCT treatment, most trial participants remained in remission and their MS had stabilized. In addition, some participants showed improvements, such as recovery of mobility or other physical capabilities. It seems as if results such as that should shift research into high gear.
Is research moving fast enough?
Why, then, do investigators seem not to have greater urgency in making HDIT/HCT treatment available in the U.S.? “If these findings are confirmed in larger studies, HDIT/HCT may become a potential therapeutic option for patients with active relapsing-remitting MS, particularly those who do not respond to existing therapies,” said Daniel Rotrosen, MD, director of NIAID’s Division of Allergy, Immunology and Transplantation.
To me, that sounds like several more years of study before researchers will be ready to ask the U.S. Food and Drug Administration to approve this treatment. I’m not a doctor. I’m not a scientist. I’m just an MS patient who’s anxious to halt the progression of my disease and to walk better. Do I need to wait another five years or more while “a large, randomized trial” is conducted?
Just venting, I guess. But scientists have been studying stem cell treatments for years and it sure seems as if we’re still crawling when we should be cruising.

I was wondering about how stem cell research was doing during the past couple of years. I guess it makes sense that there should be more years of study and tests before this treatment is available to the public. Hopefully we’ll be able to make new developments in stem cell research soon.
Over twenty years and Ocrelizumab is the best
that our “scientists ” can come up with!
It’s obvious that no one can stay on this for long
as you cannot deplete B cells completely
without major complications!
Research everyone before you take this!
I always thought from the beginning, I was diagnosed officially in 1999, Stem Cell treatment would be the answer/the cure. I know there are risks but depending on our current health status I strongly believe we should be able to make an educated decision.
I agree with you u2hearts, I think it is great that RRMS has the possibility to be treated like this, but I feel really frustrated at the lack of research toward SPMS.
Sorry now I’m venting
Another relapse-remitting? I thought stem cells were more for secondary progressive? Where are the secondary drugs?
Ocrevus, which is intended for PPMS, will hopefully be approved by the FDA when it meets on March 28. Also, I believe that some PPMS/SPMS patients may be receiving Lemtrada infusions.