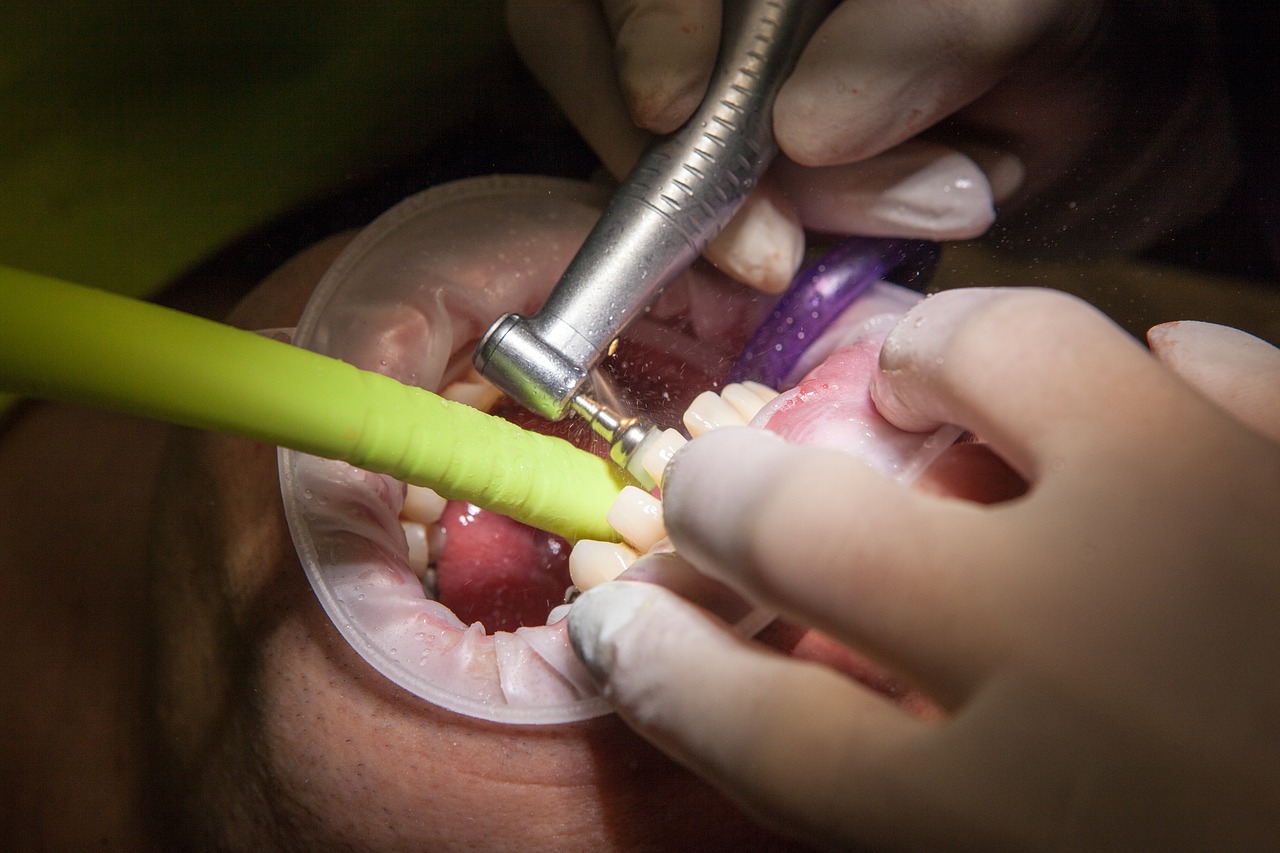
For years, some people have warned of a possible connection between multiple sclerosis and the amalgam dental fillings many of us have (or had) in our teeth. The concern has been that these fillings contain mercury, which can be toxic, especially if those fillings are removed.
In large part, these concerns have been poo-pooed. I’ve been one of the naysayers, along with the National Multiple Sclerosis Society, whose dental booklet says “there is no scientific evidence that heavy metal poisoning is responsible for either the onset or worsening of MS.” Importantly, the U.S. Food and Drug Administration had believed that “dental amalgam fillings [are] safe for adults and children ages 6 and above.”
“Had” is the key word.
Amalgam dental filling recommendations
The page on the FDA’s website containing that statement has been removed and the agency has issued a news release recommending that people in certain groups consider not using amalgam to fill their teeth. One of those groups is people with a preexisting neurological condition, such as MS.
The FDA doesn’t say point-blank, “Don’t use amalgams,” but it comes pretty close:
“… [T]he FDA strongly encourages the use of non-amalgam restorations (fillings), such as composite resins and glass ionomer cements if your dentist thinks these materials are appropriate for your affected tooth’s structure and location, and if you have no history of allergic reaction or hypersensitivity to these materials.”
The FDA adds that if you’re in one of its high-risk groups, such as someone with a neurological disease, you should discuss treatment options with your dentist and analyze the benefits versus the risks. To help you, the agency has published a brochure.
But don’t rush out and ask your dentist to remove your amalgam fillings. The FDA isn’t recommend removing or replacing an amalgam filling if it’s in good condition, unless the removal is recommended by a healthcare professional. According to the agency, doing that might briefly expose you to mercury vapor released during the removal process.
Unanswered questions
The information in the FDA’s news release leaves me with a number of unanswered questions.
- After all these years, why is the agency modifying its recommendations about amalgam fillings now? What has changed?
- Is this a prelude to broader recommendations about amalgams?
- Most importantly to me, could amalgam fillings have played a role in my MS? I had a mouth full of “silver” as a child and a young adult. Over the years, all were slowly replaced for various reasons.
- The FDA recommends that non-amalgam be used for people with a preexisting neurological condition. But what about before that condition has been diagnosed? Could I have avoided my MS by avoiding that mouth full of amalgam?
What are your questions, concerns, and experiences with dental fillings?
(This post first appeared as my column on the multiple sclerosis news today website)
(Featured image by Aleš Kartal from Pixabay)