I’ve been using a Bioness L300 for just over five years to counter my foot drop. Without the L300 strapped to my left leg, it’s difficult for me to walk more than 25 or 30 steps, even with two canes.
The L300 is a functional electronic stimulator (FES). Each time I start to lift my left leg to walk, it sends a low-intensity electrical pulse down a nerve that runs from my knee to my ankle. That pulse forces my foot to flex upward from my ankle so that my toes don’t drag. The electrical pulse replaces the signal that my brain should be sending to the nerve that my MS has blocked, and it counters my foot drop.
About six months ago, L300 manufacturer Bioness received the OK from the U.S. Food and Drug Administration to market a new model of the L300, the L300 Go. A few days ago, I had an opportunity to “test-walk” the Go, and here’s what I found.
No foot sensor needed
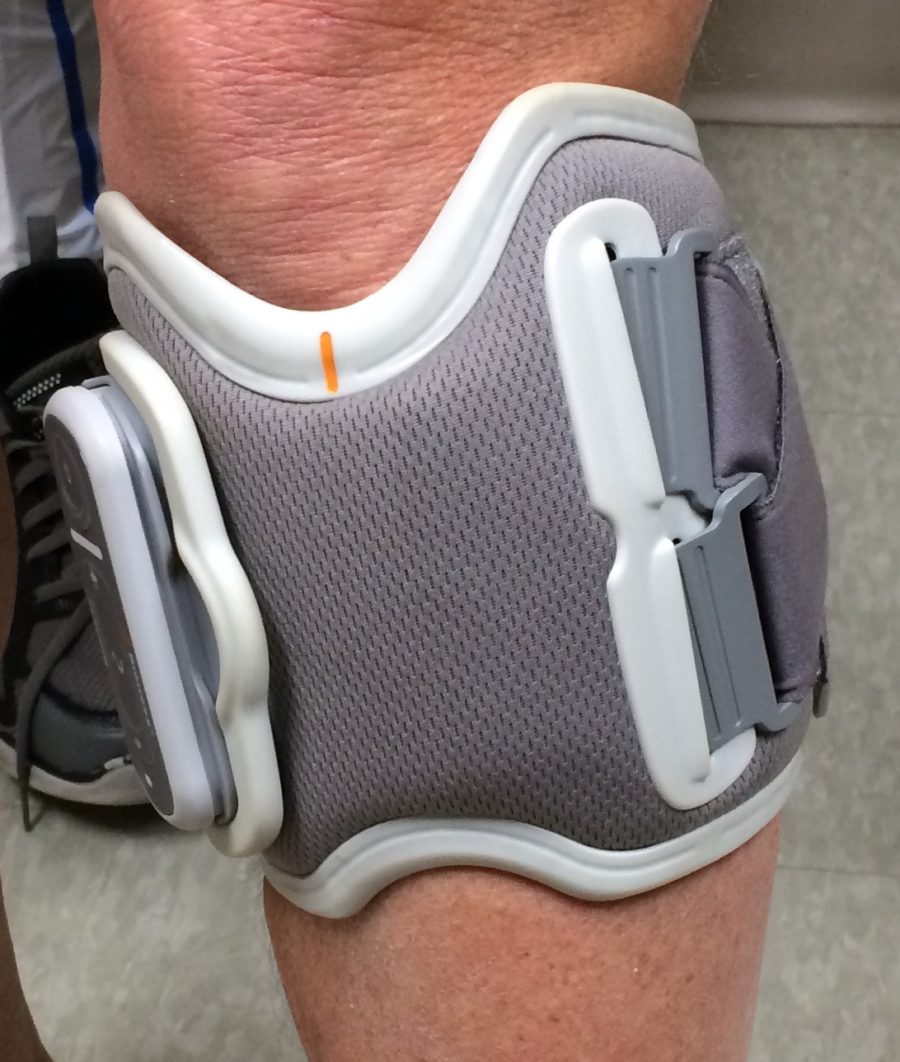
The biggest and best difference is that the L300 Go doesn’t require you to use a foot sensor in the heel of your shoe. In the original L300, the sensor is used to detect your motion as you begin to walk. It sends a Bluetooth-like signal to the cuff on your leg, telling it that you’re trying to move. The cuff then generates the pulse that stimulates the nerve in your leg and helps you to lift your foot.
That means that anytime you change shoes, you need to move the sensor to the pair that you’re going to wear. Not only is that a nuisance, there can be a lack of consistency in where you place the sensor in the shoe. Also, because insoles are different, there can be the same inconsistency in the amount of heel pressure that’s required to trigger and to end the pulse.
Naturally, you can’t use the original L300 if you’re barefoot.
3-D motion detection
Without a foot sensor, the L300 Go’s cuff detects the start of your leg motion. This is similar to the process used by the WalkAide, a competitor of the L300. Unlike the WalkAide, the L300 Go has a 3-D-motion-detection system. Not only does it detect the forward and backward motion of your leg, it also detects sideways movement and leg rotation. This allows a physical therapist to adjust the unit more precisely to make it more responsive to each patient’s unique gait. To provide this three-way detection the Go contains four electrodes, compared with the original unit’s two.
No control unit
The original L300 requires you to carry a small control unit, which you use to turn the device on and off, put it into “test mode,” and also to adjust the intensity of its pulse. With the Go, this is all done on the side of the cuff. (If you really want a foot sensor or a control unit, Bioness will sell you one. But why would you want one?)
The test walk
The Go looks and feels like the original L300. Maybe I walked a little faster with it. Maybe it triggered a little quicker. I don’t think that it allowed me to walk any further than normal. On the other hand, it was certainly nice to be able to sit in a chair without having to turn off the unit so that it wouldn’t trigger when I released the pressure on a heel sensor. Unfortunately, I forgot to test the Go walking up and down stairs. My guess, however, is that it would work better than the original unit because its trigger mechanism isn’t dependent upon heel pressure.
What’s it going to cost?
The Bioness rep told me that the Go will cost the same as the current L300. That’s about $6,200. I was also told that there would be a discount if current users wanted to upgrade, but she couldn’t say how much it would be.
Don’t count on insurance picking up the cost. Bioness apparently has had greater success recently getting insurance to pay for the L300 than when I got mine in the fall of 2012. But, it’s a fight and a lot of really good documentation is necessary. The same goes for Medicare and Medicaid. Again, don’t hold your breath waiting.
Do I plan to upgrade?
Nope, not at that price. Even with a discount, the benefit that I’d receive probably wouldn’t be enough to justify the cost of the upgrade, at least not now.
Bioness tells me that it intends to support the original L300 units for “at least the next three years.” So, I guess if my unit breaks down in 2020 or later, I may have to go for the Go.
If the L300 Go is right for you, however, Bioness expects to begin shipping it in October to patients whose doctors have given them a written order.
(This post first appeared as one of my columns on www.multiplesclerosisnewstoday.com).



Is there someway to donate or sell the old Bioness system?
Hi Sharon, I don’t know. Have you asked Bioness customer service?