(This is a sightly updated version of a column of mine that first appeared on www.multiplesclerosisnewstoday.com)
Earlier this year Bioness announced that the U.S. Food and Drug Administration had cleared its new “L300 Go” functional electronic stimulator (FES). It’s an upgrade of the original “L300” that I’ve been using for more than five years. Without the “L300” strapped to my left leg it’s difficult for me to walk more than 25 or 30 steps, even with two canes.
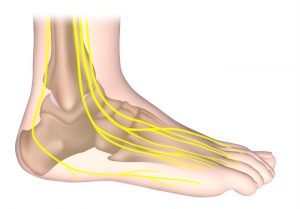
The “L300” sends a low-intensity electrical pulse down a nerve that runs from my knee to my ankle each time I begin trying to lift my left leg to walk. That pulse forces my foot to flex upward from my ankle, so my toes don’t drag. (What the docs call “foot drop“). The electrical pulse replaces the signal from my brain to my ankle that’s blocked by my MS.
to lift my left leg to walk. That pulse forces my foot to flex upward from my ankle, so my toes don’t drag. (What the docs call “foot drop“). The electrical pulse replaces the signal from my brain to my ankle that’s blocked by my MS.
When Bioness recently announced the FDA clearance of the “L300 Go” a news article was published on the Multiple Sclerosis News Today web site. That story generated several questions from readers, who wanted a better understanding of the “L300 Go” and how it’s different from the “L300.” So, I’ve been in touch with the folks at Bioness and will try to answer some of the questions that I’ve seen posted.
What’s the difference between the “L300” and the “L300 Go?”
As I understand it, the “L300 Go” allows a therapist to use 3-D motion detection system to better adjust an “L300” to make it more responsive to a person’s gait. The 3-D motion detection seems to be the most important new feature. This motion detection system also allows “L300 Go” to be used without the sensor that the “L300” requires to be placed your shoe. That means you’re able to change shoes without having to move a sensor, and even use the device barefoot! (A competitive device, called the “WalkAide,” has had this feature since it came on the market around the same time as the “L300”). The “L300 Go”also responds to motion somewhat faster than the “L300.”
I’ve been using the L300. Does FDA clearance mean that Medicare and insurance will now pay for it?
The FDA clearance was for a new product, the “L300 Go.” The “L300” was cleared by the FDA in the U.S., and received European Commission approval several years ago. The recent clearance doesn’t change the fact that, though Medicare has approved the L300 for use by spinal cord injury patients, and at least one nervous system disease, Medicare has never approved the “L300” for use by MS patients. As we all know, insurance companies aren’t likely to approve something that Medicare hasn’t approved.
Will Medicare pay for the “L300” for MS patients in the future?
A spokesperson for Bioness tells me that “veterans and their families already have access to our technology as a covered benefit through the Veteran’s Administration. We continue to work with the Centers for Medicare & Medicaid Services (CMS), as well as private/commercial insurance companies, to expand coverage criteria to include more of their beneficiaries.”
Will the price of the “L300” drop now?
I doubt it. I haven’t seen what the price will be for the “L300 Go,” (if that’s even been determined), but Bioness has said it will give a price break to current “L300” users who want to “upgrade.”
Why do you say the FDA “cleared” the “L300 Go,” rather than “approved” it?
It’s a technicality. The FDA “approves” new drugs but it “clears” the use of new medical equipment. Don’t ask me why.
I use the VA in Richmond, VA for Rehab. I had a stroke three years ago. Could you tell me who is it that works for Richmond in Bioness? I have the L200, for my arm but I want L300 Go for my leg!
Thank you so much!!!
Hi Denicia,
I don’t work for Bioness but you can get info from their Customer Service people. You can find contact info on their website: http://www.bioness.com
Ed