The advice issued last week by the U.S.-based National Multiple Sclerosis Society about COVID-19 vaccination couldn’t be clearer: “Get your vaccine as soon as it is available to you.”
The new MS Society guidelines say that the two COVID-19 vaccines currently available in the U.S., both of which use an mRNA model, are “safe and effective.” The risk of contracting severe COVID-19 far outweighs any potential vaccination risks, they add.
The guidelines say, specifically, that COVID-19 vaccines are safe for people with all forms of MS. They’re particularly important for older people with MS, people with progressive MS, those with a high level of physical disability, those with certain medical conditions – such as diabetes, high blood pressure, obesity, or heart and lung disease – for pregnant women and for Black and Hispanic people.
COVID vaccine FAQs
(Answers are based on these new guidelines.)
Are the mRNA vaccines “live” vaccines?
No, and they will not cause COVID-19.
Will the vaccines cause a relapse or increase your MS symptoms?
It’s unlikely. The vaccines might cause a fever for a day or two, which may exacerbate some MS symptoms, but symptoms should ease when the fever is reduced.
How will the vaccine affect my disease-modifying therapy (DMT), and vice-versa?
The National MS Society recommends continuing your DMT unless your healthcare provider says otherwise. Some DMTs may make the vaccine less effective, but it will still provide some protection against COVID.
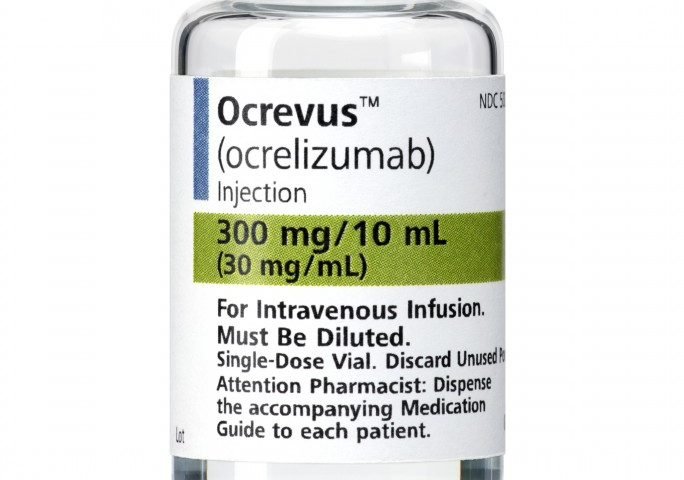
If you’re being treated with Kesimpta (ofatumumab), Lemtrada (alemtuzumab), Mavenclad (cladribine), Ocrevus (ocrelizumab), or Rituxan (rituximab), you may need to coordinate the timing of your vaccine with the timing of your DMT dose to receive the most benefit.
These recommendations were created by a group of 11 MS experts and are endorsed by the Consortium of MS Centers, along with 10 other MS organizations. They follow, by about a month, similar recommendations issued by the U.K.’s MS Society.
Are you ready to get a vaccine?
I wrote an “MS Wire” column detailing the U.K. guidelines last month. Several people have commented on that column and most can’t wait to receive a vaccine. They understand that, as the new National MS Society recommendations state, “vaccination against COVID-19 is critical for public safety and, especially, the safety of the most vulnerable among us.”
Yet, a handful of commenters wrote things like, “I am not sure if I will take it even if my doctor recommends this because it scares me that vaccine was made so fast also I heard several doctors state they will not know how long this vaccines lasts and if it will keep people from getting the virus.”
I’ve always believed that treatment decisions are individual and best made as a collaboration between patient and doctor. I continue to believe this. But I hope the MS Society’s statements that the COVID-19 vaccine is “safe and effective” and is “critical for public safety” will weigh heavily in your decision. I hope you will follow their advice to get one “as soon as it is available to you.”
I know I will.
(A version of this post first appeared as my column on the Multiple Sclerosis News Today website.)
(Featured image by torstensimon from Pixabay)