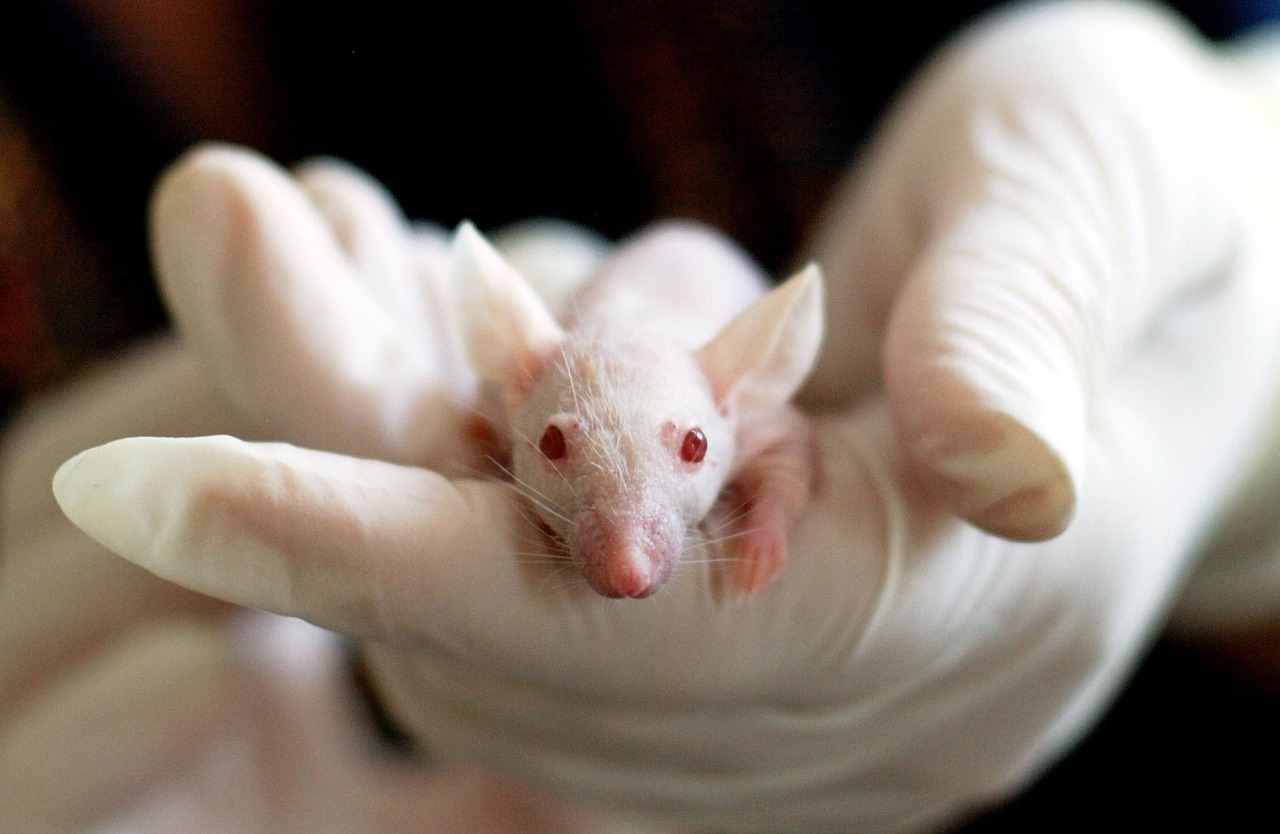
The headline on the news release that dropped into my in-box recently read: “FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs.”
Take animals out of the drug approval process? Really?
Monocolonal antibody therapies are some of the highest efficacy treatments in the multiple sclerosis (MS) arsenal. They include Tysabri (natalizumab) Lemtrada (alemtuzumab), Ocrevus (ocrelizumab, Kesimpta (ofatumumab), and Briumvi (ublituximab). (Notice the “mab” at the end of each scientific name, indicating it’s a monocolonal antibody.) I was treated with two of those, Tysabri and Lemtrada. Could these treatments haven been developed without testing drugs on animals?
My attention was immediately captured.
The U.S. Federal Drug Administration’s (FDA) news release went on to announce that animal testing will be “reduced, refined, or potentially replaced” with testing methods that the FDA considers “more effective and human-relevant.” These include predicting a potential treatment’s behavior using toxicity and cell line testing models that are based on artificial intelligence (AI). The agency also plans to promote using “lab-grown human ‘organoids’ and organ-on-a-chip systems that mimic human organs – such as liver, heart, and immune organs – to test drug safety.” It says these could reveal toxic effects that might go undetected in tests using animals. The agency calls these “New Approach Methodologies (NAMs).”
FDA calls it a “paradigm shift”
“For too long, drug manufacturers have performed additional animal testing of drugs that have data in broad human use internationally. This initiative marks a paradigm shift in drug evaluation and holds promise to accelerate cures and meaningful treatments for Americans while reducing animal use,” FDA Commissioner Martin A. Makary says in the news release. “By leveraging AI-based computational modeling, human organ model-based lab testing, and real-world human data, we can get safer treatments to patients faster and more reliably, while also reducing R&D costs and drug prices. It is a win-win for public health and ethics.”
The FDA says it plans to begin following this plan immediately when it reviews new drug applications (INDs). It adds that it encourages inclusion of NAMs data with these applications. Also in the works is incorporating safety data from other countries – ones that have similar regulatory standards to the U.S. – when it considers experimental treatments.
The FDA began working on alternative testing methods several years ago and it issued a report in January of 2021 promoting a reduction of animal testing and the use of newer testing methods. The report recognized that animal testing is necessary in many situations, but also called it expensive, time-consuming, and warned that it doesn’t always detect toxic effects that might affect humans but not animals.
Some researchers joke that “mice exaggerate, and monkeys lie.” According to a 2021 report by the U.S. National Institutes of Health, more than 90% of MS drug candidates that showed promise in animal studies failed to produce the same positive effects when tested in patients. A 2014 study published in the American Journal of Translational Research reported that less than 8% of cancer treatments make it from animal studies into a clinical setting. And according to a study published in the July 2022 issue of Acta Pharmaceutica Sinica B, only 10% of the medications in a clinical trial actually received final FDA approval.
Are we ready stop testing drugs on animals?
Are AI and other NAMs now advanced enough for us to trust these tools to replace or supplement animal testing? Before a final okay is given, I’d like to hear more from scientists, inside and outside of the FDA, as well as from the public. Yet, the news release says “the FDA and federal partners will host a public workshop later this year [emphasis added] to discuss the roadmap and gather stakeholder input on its implementation.” That sounds to me like the FDA has already made up its mind to do this. The only questions are how.
What do you think? Is it time to replace animal testing with AI?
(Image by Tibor Janosi Mozes from Pixabay)
(A version of this post first appeared on the Rare Disease Advisor website)